BFI INNOVATION
FULL STACK
A high-velocity translational ecosystem that bridges research and commercialization

our vision
To revolutionize India’s biomedical innovation landscape by creating a seamless, all-under-one-roof ecosystem that connects ideation, prototyping, validation, and commercialization.
Powered by NAMAH’s cutting-edge facilities, the expertise of 6 partner medical colleges, real-world evaluation through the Sandbox network, and market guidance from the Product Promotion Team, this philanthropic initiative empowers innovators to transform bold ideas into clinically validated, market adopted healthcare solutions - faster, smarter, and at a global standard.
Powered by NAMAH’s cutting-edge facilities, the expertise of 6 partner medical colleges, real-world evaluation through the Sandbox network, and market guidance from the Product Promotion Team, this philanthropic initiative empowers innovators to transform bold ideas into clinically validated, market adopted healthcare solutions - faster, smarter, and at a global standard.

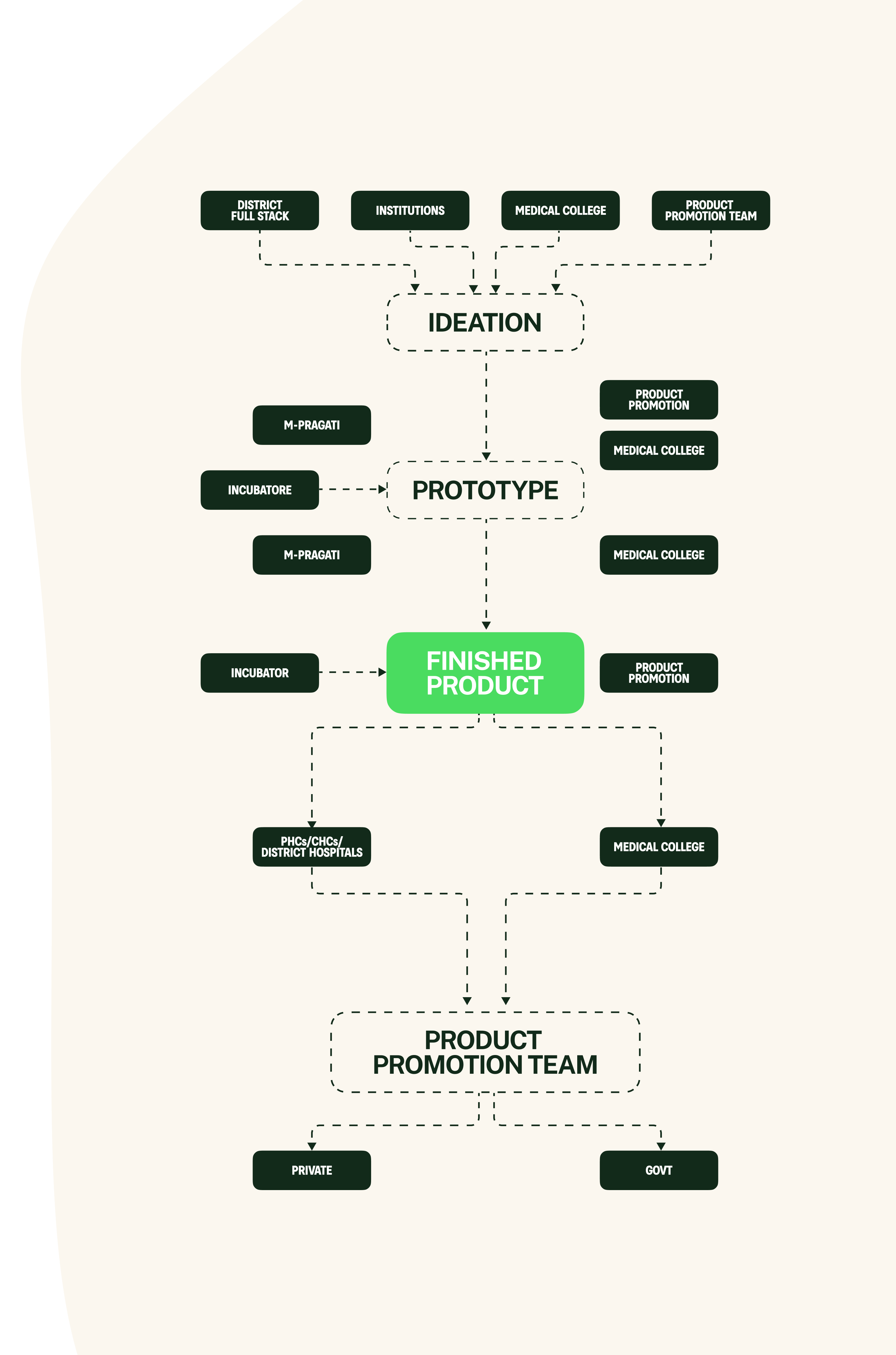
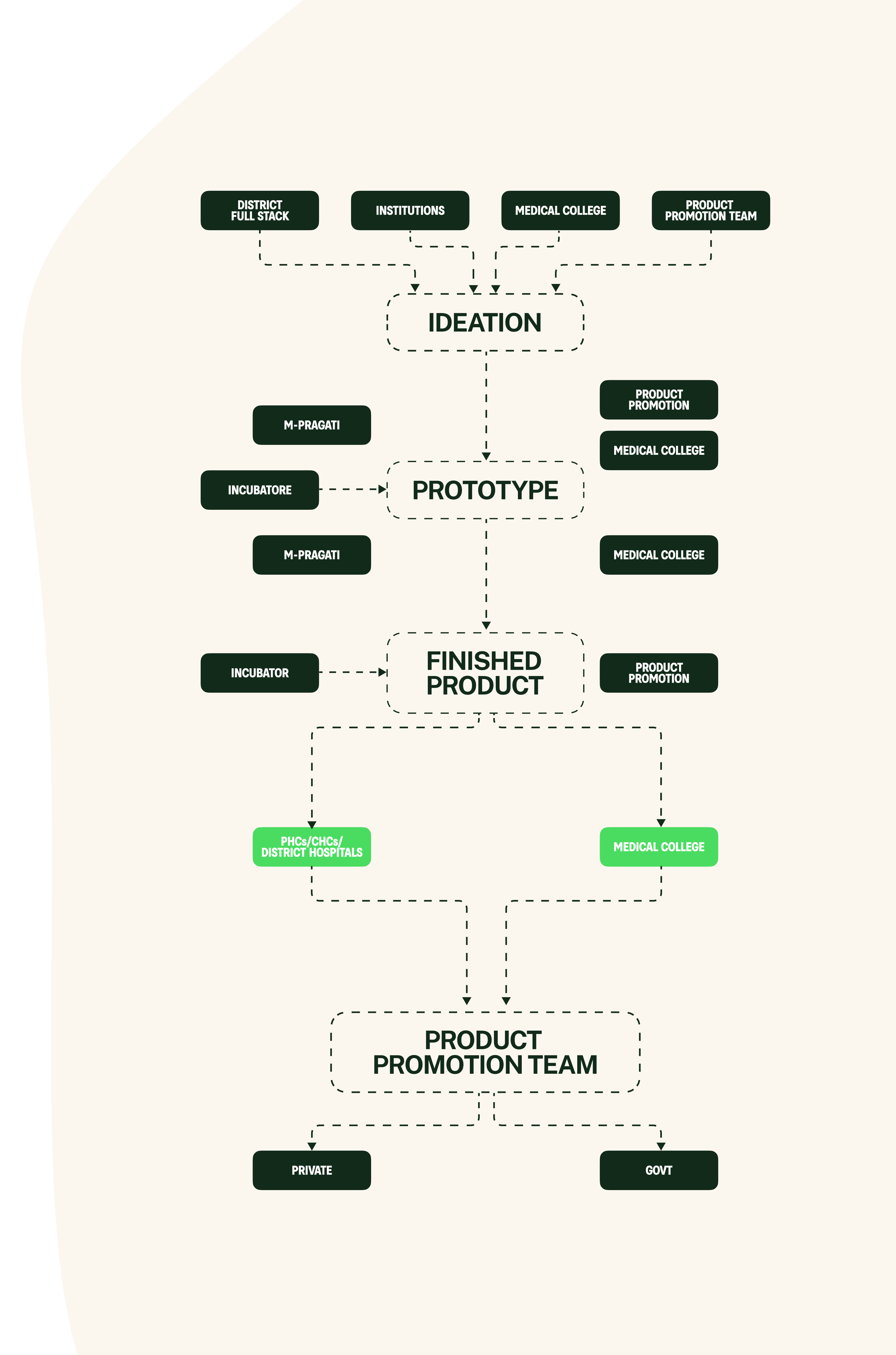
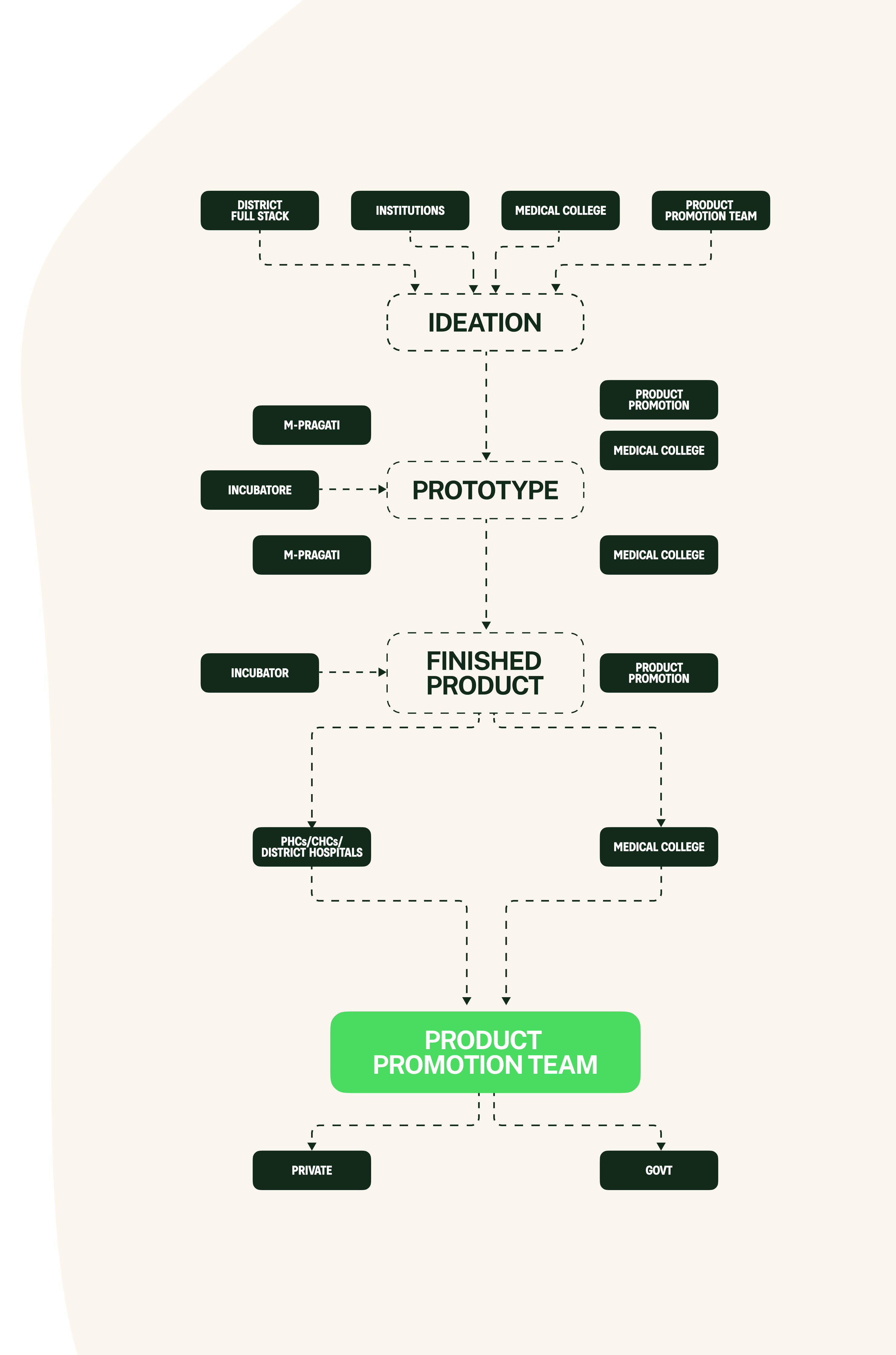
The composition
bfi innovation full stack includes:
%20(1).png)
.png)

More Programmes
What Makes BFI innovation full stack Unique
End-to-End Acceleration
Seamlessly supports the entire product journey from idea to market.

Reduced Time to Market
Integrated facilities drastically cut development and approval timelines.

All Under One Roof
R&D, prototyping, validation, and regulatory services co-located for efficiency.

No Facility Cost
Innovators gain access to state-of-the-art infrastructure at zero usage fee.
Innovator Ownership
Equity and royalty fully retained by the innovator.
Market Readiness
Dedicated product promotion team ensures smooth market adoption and scale-up.
Expert Evaluation
Continuous guidance and review by a multidisciplinary team across partner medical colleges.
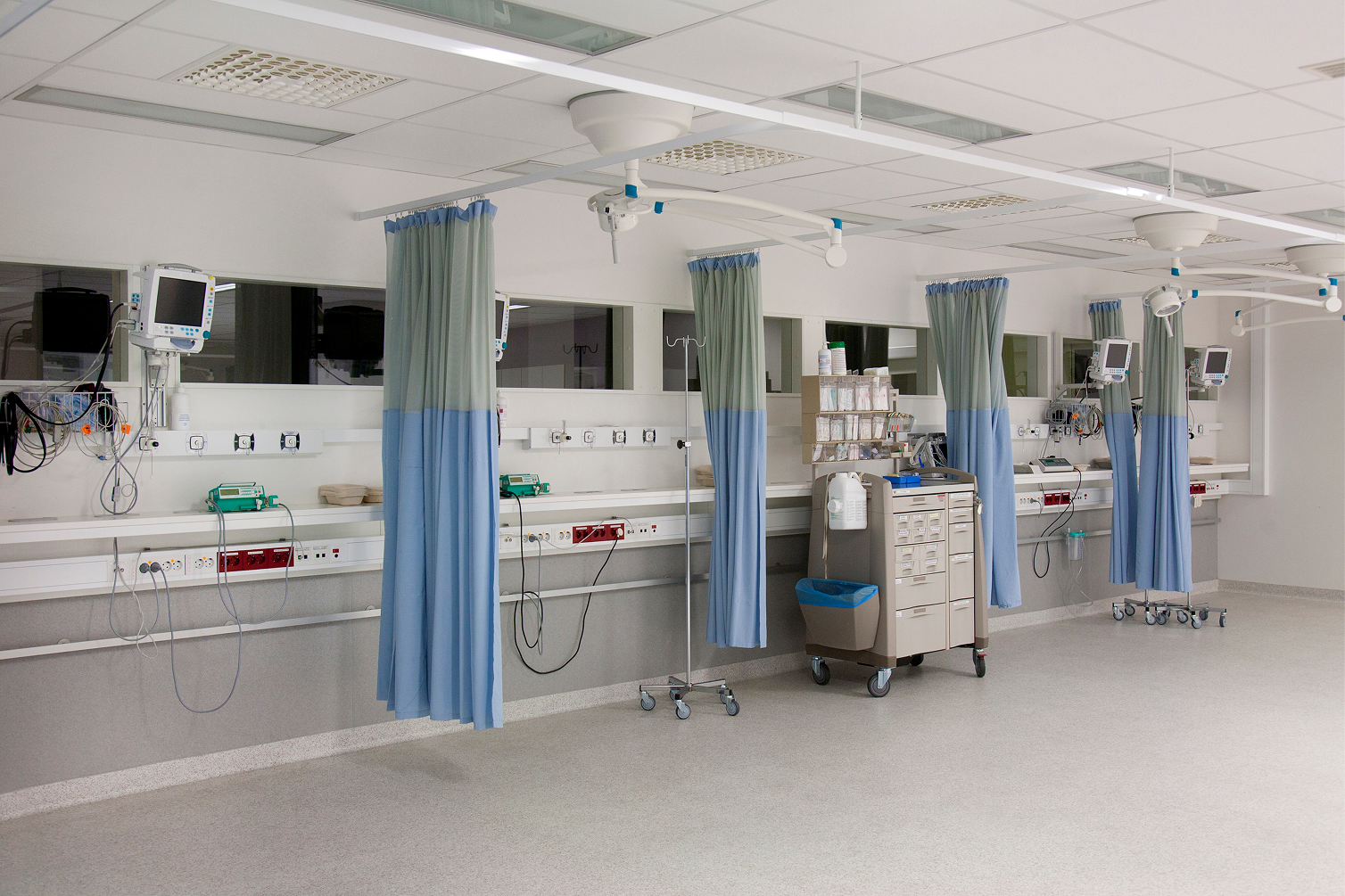
Clinical Sandbox
Real-world simulation and large-scale clinical validation under controlled settings.

Animal Testing Facility
Integrated preclinical testing support through BRIC-THSTI collaboration.
.svg)
.png)

.png)
.png)

.png)
.png)
.png)
.png)
.png)
.png)

.svg)